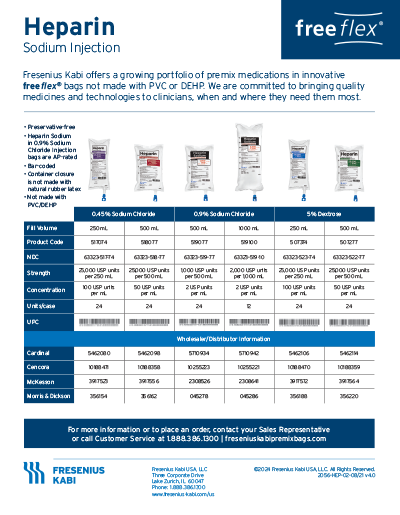
Heparin
Sodium Injection








0.45% Sodium Chloride
0.9% Sodium Chloride
5% Dextrose
Fill Volume
250 mL
500 mL
500 mL
1,000 mL
250 mL
500 mL
Product Code
517074
518077
519077
519100
507374
507277
NDC
63323-517-74
63323-518-77
63323-519-77
63323-519-10
63323-523-74
63323-522-77
Strength
25,000 USP units
per 250 mL
25,000 USP units
per 500 mL
1,000 USP units
per 500 mL
2,000 USP units
per 1,000 mL
25,000 USP units
per 250 mL
25,000 USP units
per 500 mL
Concentration
100 USP units
per mL
50 USP units
per mL
2 USP units
per mL
2 USP units
per mL
100 USP units
per mL
50 USP units
per mL
Units/case
24
24
24
24
24
24
UPC






Wholesaler/Distributor Information
Cardinal
5462080
5462098
5710934
5710942
5462106
5462114
Cencora
10188471
10188358
10255223
10255221
10188470
10188359
McKesson
3917523
3917556
2308526
2308641
3917572
3917564
Morris & Dickson
356154
356162
045278
045286
356188
356220
- Preservative-free
- Heparin Sodium in 0.9% Sodium Chloride Injection bags are AP-rated
- Bar-coded
- Container closure is not made with natural rubber latex
- Not made with PVC/DEHP
Heparin Sodium Injection
Product Insert
View PDF