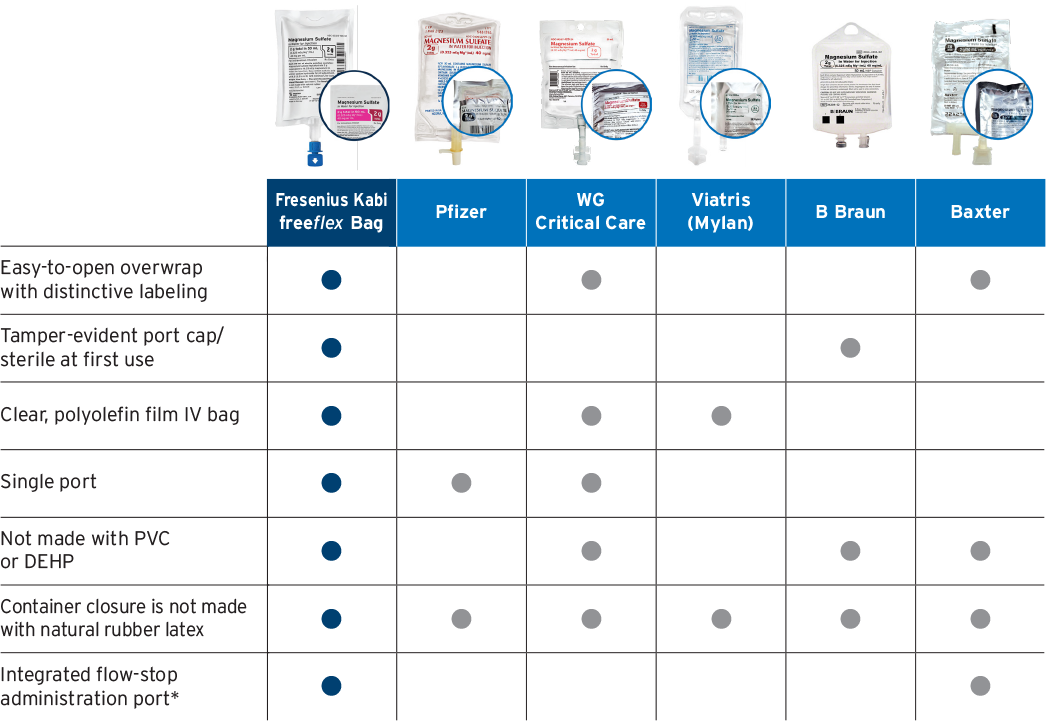
Premix: An ISMP best practice

Best practice
According to the Institute for Safe Medication Practices (ISMP): To the extent possible, using commercially manufactured parenteral products over manually compounded sterile preparations is a Best Practice for Sterile Compounding.1
Patient safety
Compounding errors to avoid
- Incorrect dose or concentration (58%)
- Incorrect base solution (51%)
- Incorrect base solution volume (43%)
- Incorrect reconstitution of a drug (volume or diluent) (36%)
- Incorrect drug (35%)
- Expired drug, base solution, or compounded sterile preparation (16%)
- Omission of a drug (5%)
Greater efficiency
The use of premix could help save pharmacy staff time spent in compounding, freeing them to do more
patient-focused tasks.3
Less waste
The use of premix medications may help reduce product waste due to the longer shelf life of premix over compounded products.3
Clinician safety
The use of premix may help reduce needlesticks and accidental drug exposure by reducing the use of syringes to add medication to IV bags in the compounding process.
References
1. 2022 ISMP Guidelines for Sterile Compounding and the Safe Use of Sterile Compounding Technology, page 17.
2. Institute for Safe Medication Practices, October 22, 2020. ISMP Survey Provides Insights into Pharmacy Sterile Compounding Systems and Practices. Accessed at https://home.ecri.org/blogs/ismp-news/ismp-survey-on-sterile-compounding-in-pharmacies-reveals-gaps-challenges on January 3, 2024.
3. AJ Loeb, DA Fishman, TR Kochis. Premixed intravenous admixtures: a critical challenge for hospital pharmacy. Am J Hosp Pharm 1983 Jun;40(6):1041-3. Accessed January 3, 2024.